How it works
Orbera® is placed endoscopically to help fill the stomach and encourage portion control. It is a straight-forward, nonsurgical procedure that helps patients learn healthy habits when combined with diet, exercise, and behavioral counseling. Six months later, the balloon is removed. Watch the video to see how Orbera® works.

STEP 1
Deflated Orbera® balloon is inserted endoscopically by a trained gastroenterologist or surgeon.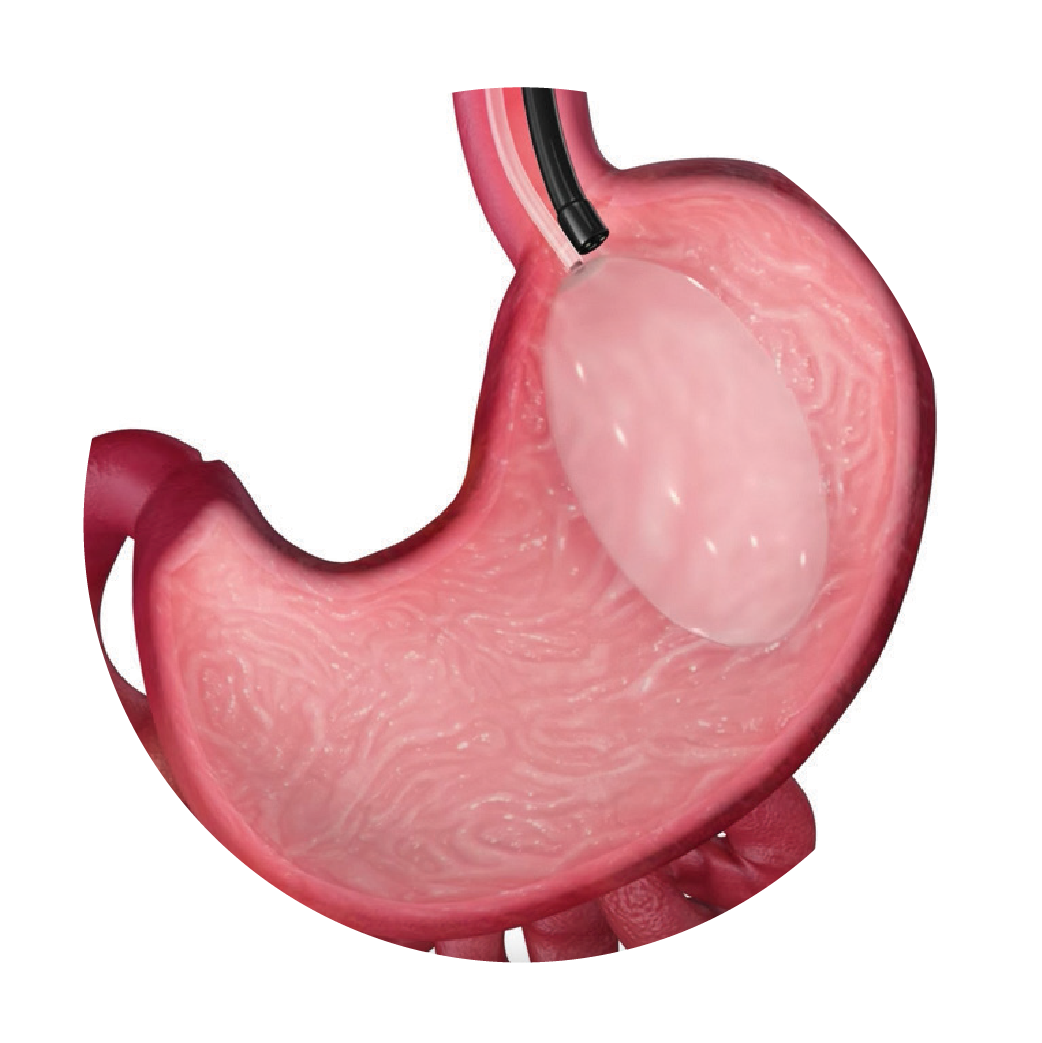
STEP 2
The balloon is then filled with a sterile saline solution to a volume of 400-700 cc. It is important to use sterile saline and an aseptic technique to minimize the risk of introducing microorganisms to the balloon, which could lead to inflation of the balloon after placement.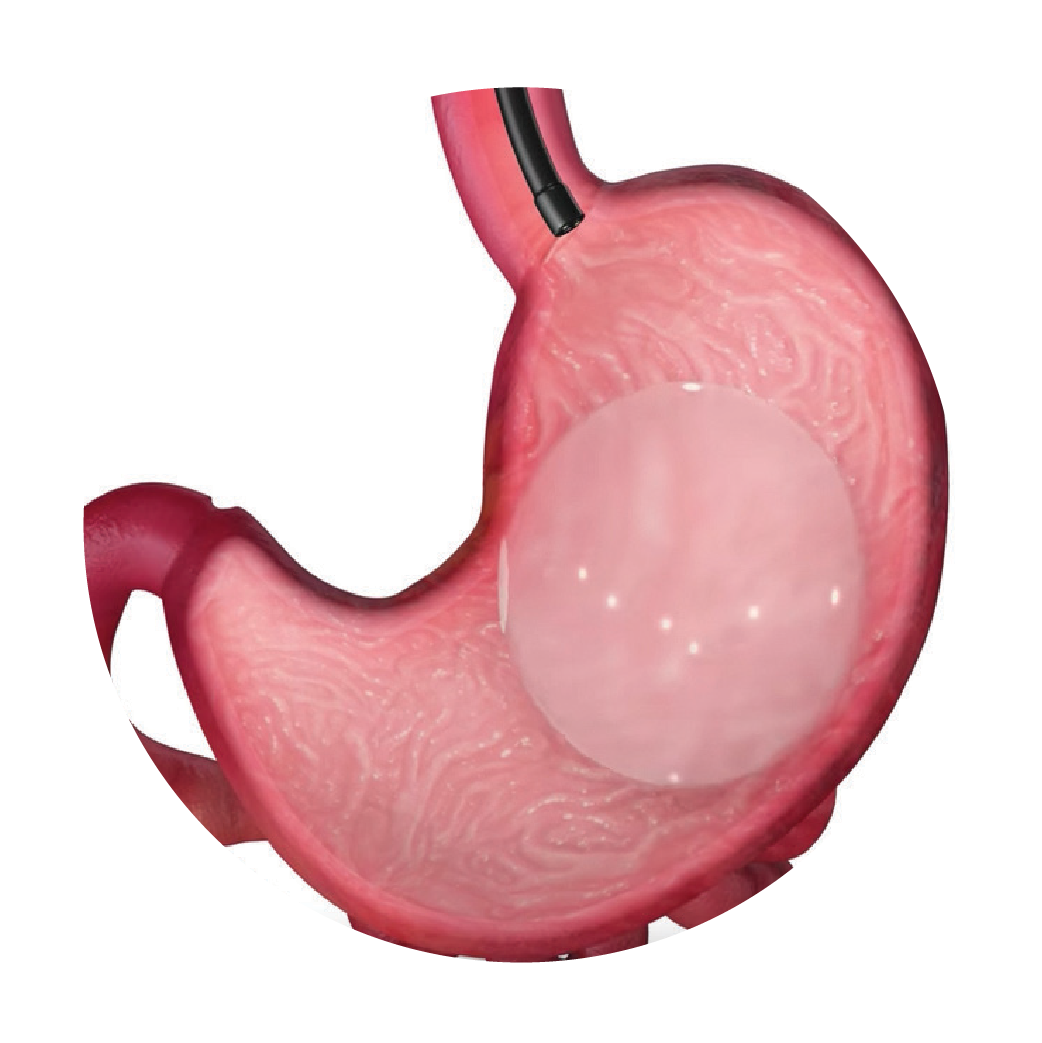
STEP 3
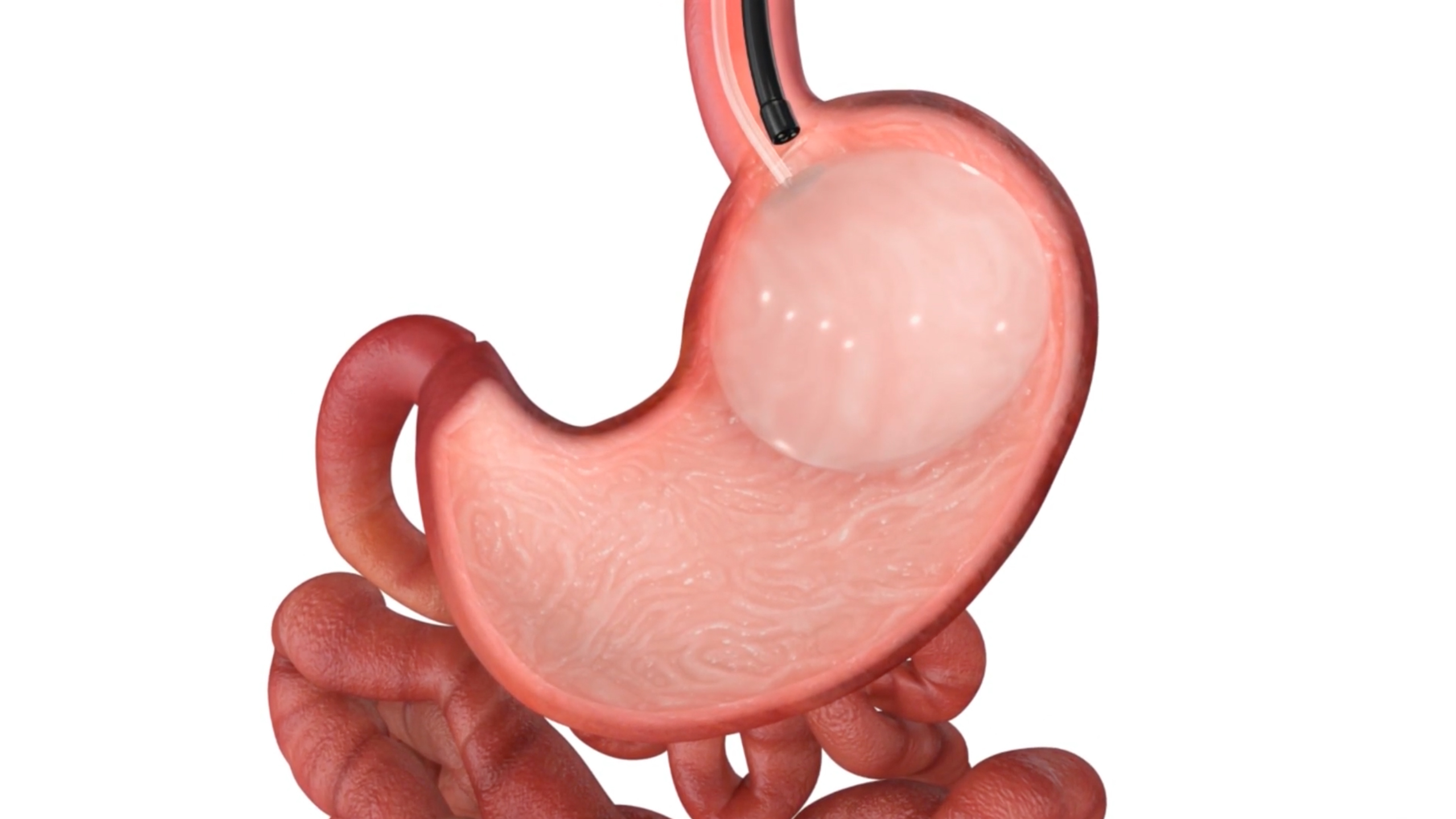
The smooth, soft, spherical Orbera® balloon takes up space in the stomach and delays gastric emptying, aiding in weight loss.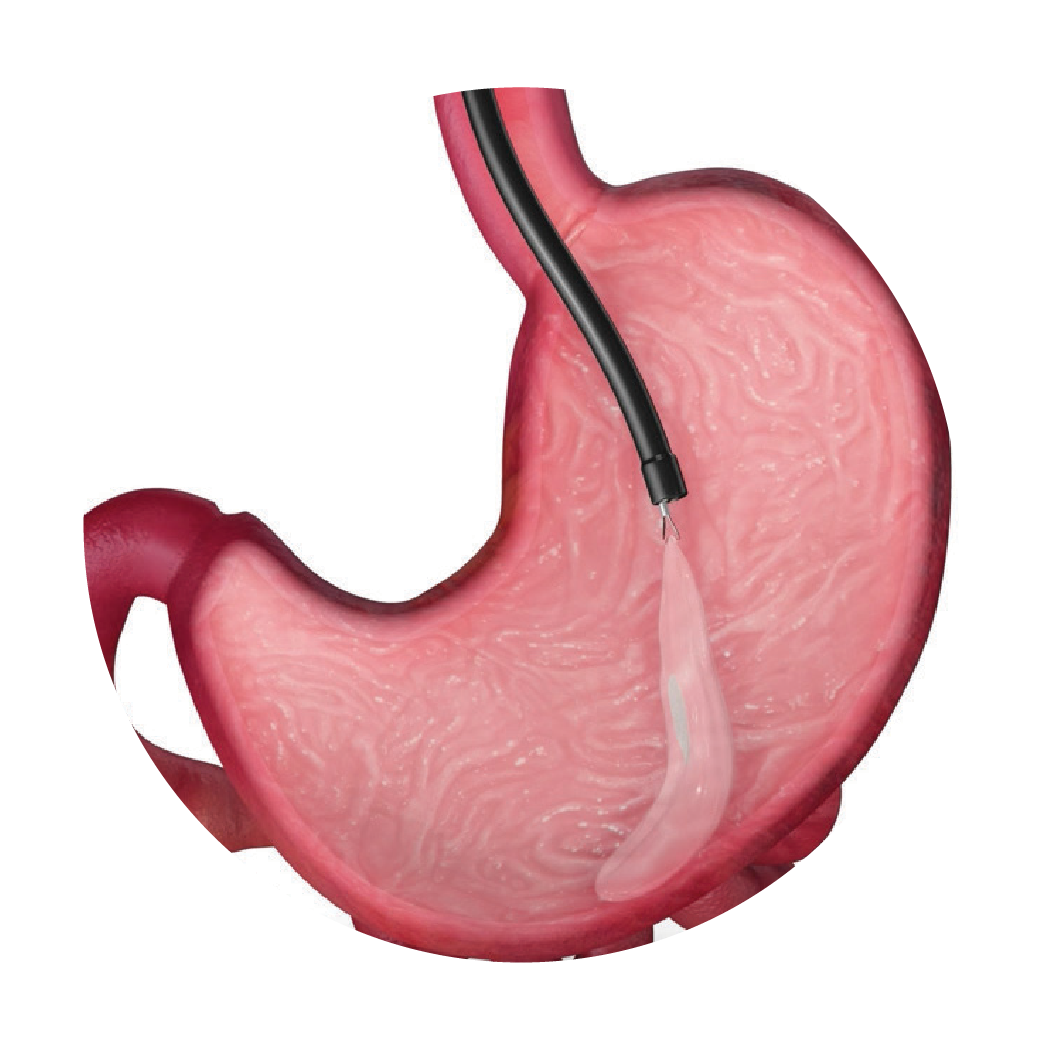
STEP 4
After six months, the Orbera® balloon is removed endoscopically. The removal should be scheduled in advance to avoid the chance of prolonged dwell time. Certain risks increase with extended balloon dwell time, such as balloon deflation which can result in a blockage in the stomach or intestines in rare cases.
The world’s #1 weight loss balloon
Orbera® was commercially launched outside the U.S. in 2004 and is now used
to assist patients with weight loss in over 80 countries.

Orbera® has been approved by the FDA for weight loss in patients with obesity (BMI 30-40 kg/m2).
Orbera® is a reversible nonsurgical weight loss procedure. Future treatment options are preserved.

Millions of pounds have been lost with Orbera®, with over 400,000 of Apollo’s gastric balloons distributed worldwide.

Orbera® is the global leading weight loss balloon and has helped thousands meet their weight loss goals for over 20 years.
Clinical legacy
The Orbera® balloon was designed to fulfill the consensus criteria of a multi-disciplinary international physician collaboration, which listed these features of an ideal intragastric balloon effective in producing weight loss.
- Spherical shape with a smooth, soft surface to reduce chance of ulcers.
- Filled with sterile saline rather than air to fill the stomach and reinforce portion control.
- May be filled to a volume of 400-700cc.
- Radiopaque valve to support visualization within the stomach.

Clinical evidence
Since Orbera®’s inception, there have been over 250 peer-reviewed Orbera® publications around the globe including data on over 8,000 patients. Retrospective and pivotal studies show, time and again, that Orbera® is safe and effective, and helps patients lose the weight and keep the weight off even after the device is removed. Keeping the weight off requires that the patient maintains healthy habits after the balloon is removed. This should be reinforced to the patient for optimal results.
-
3.1x
More weight loss than diet and exercise alone.1
-
400,000
Devices distributed worldwide in over 80 countries.
-
20
years’ experience worldwide.
1 Orbera® US FDA Pivotal Study.
Important Orbera® Intragastric Balloon System Safety Information for Healthcare Professionals:
The Orbera® Intragastric Balloon System is a weight loss aid for adults suffering from obesity who have tried other weight loss programs, such as following supervised diet, exercise, and behavior modification programs, but who were unable to lose weight and keep it off.
To receive Orbera® a patient must be willing to also follow a 12-month program, beginning with the placement of Orbera® and continuing for six months after, that includes a healthy diet and exercise plan. If the diet and exercise program is not followed, patients may not experience significant weight loss results.
After placement, the patient and physician should maintain communication as the patient acclimates to the balloon. Early nausea and vomiting are expected. In cases where the patient experiences severe pain or vomiting they should contact their physician immediately. In rare cases, severely delayed gastric emptying can lead to perforation. If unrecognized, this can lead to sepsis and death. In such cases, the balloon must be removed immediately.
Potential adverse events include nausea, vomiting, balloon deflation, hyperinflation after the procedure, delayed gastric emptying, perforation and death.
For a full listing of adverse events, refer to the instructions for use at apolloendo.com/dfus.
Orbera® is placed for no more than six months. Extending the dwell time increases the risk of certain adverse events, most notably balloon deflation, which can lead to a blockage in the stomach or intestines. Some patients are ineligible to receive Orbera®. Patients must not receive Orbera® if they are pregnant or planning to become pregnant within six months’ time, or breastfeeding.
All patients need to review and understand all the complications and risks before undergoing any procedure. At all times physicians will act as an independent agent and use their independent medical judgement to determine whether any procedure is in the best interest of any particular patient.
There is a patient information booklet available online at apolloendo.com/dfus.
CAUTION: Rx only.

